Have you ever wondered what makes up the things around you? What if I told you that everything, from the chair you’re sitting on to the air you breathe, is made up of tiny, fundamental particles called atoms? And within those atoms lie even tinier particles – protons, neutrons, and electrons! These building blocks, these tiny, powerful forces, determine the very nature of matter. Let’s dive into the fascinating world of protons, neutrons, and electrons, and explore a practice worksheet answer key to help you better understand these fundamental particles.
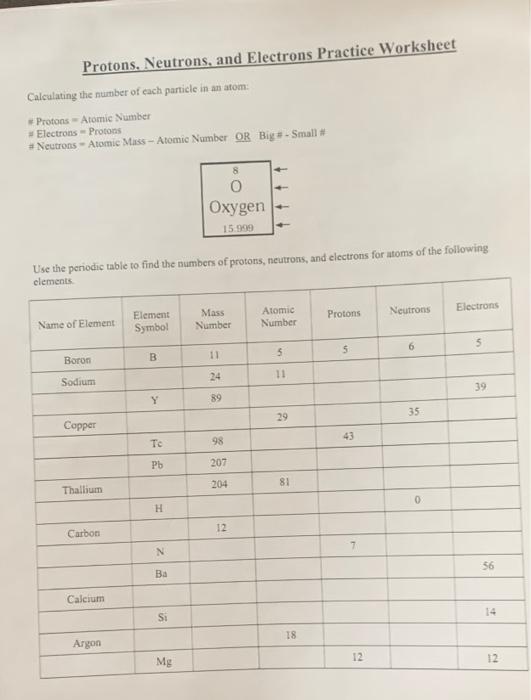
Image: www.chegg.com
The concept of the atom, the smallest unit of an element, is a foundational principle in chemistry. Understanding its components is crucial for mastering the subject. These subatomic particles, protons, neutrons, and electrons, are essential for comprehending how elements interact and form various substances. This practice worksheet will guide you through understanding their roles, charges, locations within the atom, and how they contribute to an atom’s identity.
The Building Blocks of Matter: Protons, Neutrons, and Electrons
Protons: The Positive Charge
Protons, denoted by the symbol “p,” are positively charged particles residing within the atom’s nucleus. The nucleus is like the atom’s core, a dense and tightly packed region that holds most of the atom’s mass. Each proton carries a single unit of positive charge, represented by a “+” sign. The number of protons within an atom defines its atomic number.
For instance, a hydrogen atom has only one proton, making its atomic number 1. Carbon, with six protons, has an atomic number of 6. The atomic number is unique to each element, and it acts as a sort of “identification card” for that element.
Neutrons: The Neutral Force
Neutrons, symbolized by “n,” are neutral particles also found within the nucleus. They have no charge, hence the name “neutron.” They play a crucial role in stabilizing the atom’s nucleus. Unlike protons, the number of neutrons can vary within a single element. These variations, called isotopes, have the same number of protons but different numbers of neutrons, leading to different atomic masses.
For example, Carbon-12 has 6 protons and 6 neutrons, while Carbon-14 has 6 protons and 8 neutrons. These variations impact their radioactive properties and contribute to the diverse behaviors of elements in the natural world.

Image: katesingletptriple.blogspot.com
Electrons: Negative and Orbiting
Electrons, symbolized by “e,” are negatively charged particles orbiting the nucleus in a cloud-like region called the electron cloud. Their negative charge is equal in magnitude but opposite in sign to the protons’ positive charge. Electrons are much smaller than protons and neutrons and carry a single unit of negative charge, represented by a “-” sign.
The arrangement of electrons within the electron cloud determines an atom’s chemical properties and its tendency to form chemical bonds with other atoms. These electrons are constantly moving and are responsible for the atom’s interactions with other atoms.
Practice Worksheet: Unlocking the Secrets of the Atom
Here is a practice worksheet focusing on protons, neutrons, and electrons:
Section 1: Identifying Atoms
Instructions: Complete the table below based on the information provided about each element.
| Element | Number of Protons | Number of Neutrons | Atomic Number |
|---|---|---|---|
| Carbon | 6 | 6 | 6 |
| Oxygen | 8 | 8 | 8 |
| Helium | 2 | 2 | 2 |
| Nitrogen | 7 | 7 | 7 |
Answer Key:
- Carbon: Atomic Number 6; 6 protons, 6 neutrons
- Oxygen: Atomic Number 8; 8 protons, 8 neutrons
- Helium: Atomic Number 2; 2 protons, 2 neutrons
- Nitrogen: Atomic Number 7; 7 protons, 7 neutrons
Section 2: Isotopes and Atomic Mass
Instructions: Calculate the atomic mass of the following isotopes.
| Isotope | Number of Protons | Number of Neutrons | Atomic Mass |
|---|---|---|---|
| Carbon-12 | 6 | 6 | 12 |
| Carbon-14 | 6 | 8 | 14 |
| Uranium-235 | 92 | 143 | 235 |
| Uranium-238 | 92 | 146 | 238 |
Answer Key:
- Atomic mass is the sum of protons and neutrons.
- Carbon-12: Atomic Mass = 6 + 6 = 12
- Carbon-14: Atomic Mass = 6 + 8 = 14
- Uranium-235: Atomic Mass = 92 + 143 = 235
- Uranium-238: Atomic Mass = 92 + 146 = 238
Section 3: Charge and Neutral Atoms
Instructions: Use the information provided to determine the charge of the following ions.
| Ion | Number of Protons | Number of Electrons | Charge |
|---|---|---|---|
| Sodium ion (Na+) | 11 | 10 | +1 |
| Chloride ion (Cl-) | 17 | 18 | -1 |
| Calcium ion (Ca2+) | 20 | 18 | +2 |
| Oxide ion (O2-) | 8 | 10 | -2 |
Answer Key:
- Charge is determined by the difference between protons and electrons. A positive charge means more protons, while a negative charge means more electrons.
- Sodium Ion (Na+): 11 protons (positive) – 10 electrons (negative) = +1 charge
- Chloride Ion (Cl-): 17 protons (positive) – 18 electrons (negative) = -1 charge
- Calcium Ion (Ca+2): 20 protons (positive) – 18 electrons (negative) = +2 charge
- Oxide Ion (O-2): 8 protons (positive) – 10 electrons (negative) = -2 charge
Section 4: Connecting the Dots
Instructions: Match the following descriptions to the corresponding subatomic particle.
- Positively charged, found in the nucleus
- Negatively charged, orbit the nucleus
- Neutral, found in the nucleus
- Defines the element’s atomic number
- Contributes to the atom’s atomic mass
Answer Key:
- Proton
- Electron
- Neutron
- Proton
- Both protons and neutrons
Section 5: The Big Picture
Instructions: Explain the relationship between the number of protons, neutrons, and electrons in determining an atom’s identity and properties.
Answer Key:
- The number of protons defines the element. For example, all carbon atoms have 6 protons, while all oxygen atoms have 8 protons.
- The number of neutrons can vary within an element, creating isotopes. These variations influence the atomic mass and radioactivity of the element.
- The number of electrons determines the atom’s charge and its chemical properties. Atoms with an equal number of protons and electrons are neutral. Atoms with more protons than electrons are positively charged (cations), and atoms with more electrons than protons are negatively charged (anions). The arrangement of electrons influences how the atom forms bonds with other atoms, driving chemical reactions.
Beyond the Basics: Diving Deeper into the World of Subatomic Particles
The practice worksheet provides a solid foundation in understanding protons, neutrons, and electrons. However, the world of subatomic particles is far more intricate and fascinating!
For instance, recent discoveries reveal that protons and neutrons themselves are made up of even smaller particles called quarks. These fundamental particles, along with their interactions governed by the strong force, play a critical role in holding the nucleus together.
Furthermore, the field of particle physics explores a vast array of particles, including leptons, bosons, and hadrons, uncovering the intricate mechanisms of the universe and unlocking the mysteries of the fundamental forces that govern interactions at the subatomic level.
Protons Neutrons Electrons Practice Worksheet Answer Key
Final Thoughts: A Journey of Discovery
Understanding protons, neutrons, and electrons is the foundation for exploring the world of chemistry. This practice worksheet provides a solid starting point, equipping you with the basic knowledge and skills needed for further exploration. Remember that the study of subatomic particles is a dynamic field, with constant discoveries and advancements pushing the boundaries of our understanding.
By mastering the basics, you can delve deeper into the intricate world of atoms and explore the fascinating phenomena that shape our world. The journey of discovery is ongoing, and the more you learn about these fundamental particles, the better equipped you will be to understand the world around you and the universe itself!






